Abstract
Background: Epcoritamab is a novel, subcutaneous (SC) CD3xCD20 bispecific antibody that has been shown to be efficacious in patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) as monotherapy. The phase 1/2 EPCORE NHL-1 (NCT03625037) study evaluated epcoritamab in patients with R/R LBCL who received at least 2 prior lines of systemic therapy. Patients in the trial were heavily pretreated with a median of 3 (range, 2-11) prior lines of therapy, 39% had prior chimeric antigen receptor T-cell (CAR T) therapy, 61% had primary refractory disease, and 83% were refractory to the last line of therapy. With a median follow-up of 10.7 months, the overall response rate (ORR) by independent review committee based on Lugano criteria was 63%, with a complete response (CR) rate of 39%; median overall survival (OS) was not reached (Thieblemont et al, EHA 2022). No head-to-head data have compared SC epcoritamab with chemoimmunotherapy (CIT). This study conducted an unanchored, matching-adjusted, indirect comparison of the efficacy of SC epcoritamab as reported in the EPCORE NHL-1 trial with that of CIT as reported in the SCHOLAR-1 study (Neelapu et al, Blood Adv 2021), which is the largest reported outcomes study in patients with R/R LBLCL treated with CIT.
Methods: Published OS data for CIT from SCHOLAR-1 and ORR and CR rate based on investigator-assessed International Working Group criteria were used to conduct this matching-adjusted, indirect treatment comparison versus epcoritamab. Patients with prior exposure to CAR T therapy enrolled in EPCORE NHL-1 were excluded in this analysis because the SCHOLAR-1 study only included patients without prior exposure to CAR T therapy. Analysis was conducted by adjusting for imbalances in the following baseline characteristics between individual patient-level data from EPCORE NHL-1 and aggregate data from SCHOLAR-1: age 65 years or older, sex, primary refractory disease, disease refractory to at least 2 consecutive lines of therapy, Eastern Cooperative Oncology Group performance status, relapse within 12 months of autologous stem cell transplant, and disease stages III and IV. Propensity score weights resulting from the adjustment were applied to estimate differences in risk for ORR and CR rate for epcoritamab versus CIT, and weighted Cox proportional hazards models were used to estimate the OS HR between the 2 comparators. Adjustment weights were truncated at 1% and 99% of their distribution to reduce occurrence of extreme weights.
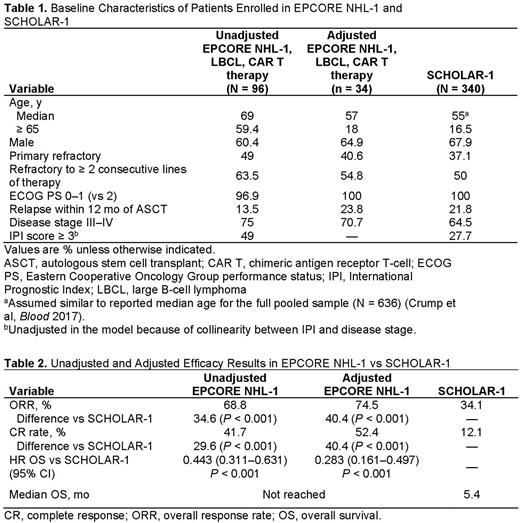
Results: Patients without prior exposure to CAR T therapy enrolled in the EPCORE NHL-1 trial (N = 96) were mostly 65 years and older, men, had stage III or IV disease, had a history of primary refractory disease, and were refractory to at least 2 consecutive lines of therapy (Table 1). After adjustment, imbalances of these baseline characteristic variables between EPCORE NHL-1 and SCHOLAR-1 were mitigated (Table 1). Before adjustment, the ORR, CR rate, and OS HRs for epcoritamab were 68.8%, 41.7%, and 0.443 (vs CIT), respectively, for patients naive to CAR T therapy (Table 2). After adjustment, compared with CIT, patients receiving epcoritamab had a higher adjusted ORR (74.5% vs 34.1%; difference in risk, 40.4%; P < 0.001) and CR rate (52.4% vs 12.1%; difference in risk, 40.4%; P < 0.001). After adjustment, patients treated with epcoritamab also had improved OS versus CIT (HR 0.283, 95% CI: 0.161-0.497; P < 0.001). The median OS for CIT was 5.4 months, whereas the median OS of epcoritamab had not yet been reached with a median follow-up of 10.7 months.
Limitations: Although this study adjusts for confounding variables between trials, outcomes are still subject to a higher level of uncertainty than what would have occurred if epcoritamab and comparators were studied within a randomized controlled trial. In addition, adjustments are based on the aggregate proportions of patient characteristics so that the associations between survival and baseline factors on the study level may be different than they would have been on the patient level.
Conclusions: Findings from this cross-study analysis suggest that epcoritamab demonstrated significantly better efficacy in a highly refractory LBCL population versus CIT. Despite lack of an available head-to-head comparison, this study contextualizes the epcoritamab data and underscores the therapeutic potential of epcoritamab.
Disclosures
Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria. Wang:AbbVie: Current Employment, Current equity holder in publicly-traded company. Mutebi:Genmab: Current Employment, Current equity holder in publicly-traded company. Alshreef:AbbVie: Current Employment, Current equity holder in publicly-traded company. Davies:AbbVie: Current Employment. Wetering:AbbVie: Research Funding; OPEN Health: Current Employment. Chirikov:BMS: Consultancy, Research Funding; OPEN Health: Current Employment; AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal